Theresa Gabrielli
July 29, 2025
Researchers in the Miqin Zhang lab at UW Materials Science & Engineering have developed a promising new way to deliver messenger ribonucleic acid (mRNA) to cancer cells. Their novel polymer platform, a new nanoparticle comprised of perfluoroheptanoic acid (PFHA), polyethyleneimine (PEI), heparin (HP) and mRNA, shows enormous potential to outperform the current standard platform, lipid nanoparticles (LNPs).
If you’ve ever gotten a COVID-19 vaccine, you may be familiar with the use of mRNA as a tool in health care. As a messenger, mRNA can give your body instructions to create proteins found in a particular pathogen, teaching your immune system to recognize that protein and create antibodies to fight it off.
Recent advances in medicine have found that this same concept has the ability not only to prevent disease but also to treat it. The trick is to safely get the mRNA in the right place to pass on the correct message. Because mRNA is delicate, the creation of delivery platforms capable of ferrying mRNA to its destination without degrading or damaging the message is essential.
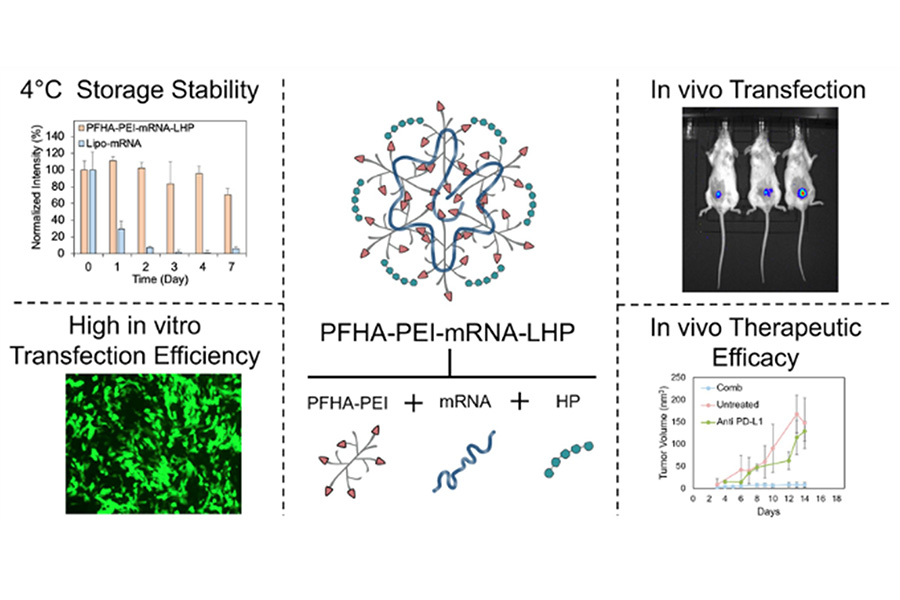
A biopolymer nanoparticle platform enables mRNA delivery with high transfection efficiency, antitumor efficacy, and cold-chain–free stability.
In a paper published July 24 in Nanoscale Horizons, Zhang’s team details how their unique combination of fluorination and heparinization in PFHA-PEI-mRNA-HP enhances mRNA delivery efficiency by improving stability, cellular uptake, and targeting.
For many years, the go-to standard delivery platform has been LNPs. Unfortunately, LNPs require extremely cold temperatures to protect their mRNA payloads, making storage and transportation costly. The formulas used to create LNPs are complicated and labor-intensive, introducing more opportunities for human error. They also have potential to damage the healthy tissue around a tumor, a side effect known as toxicity.
In testing, PFHA-PEI-mRNA-HP improved upon LNPs’ many shortcomings by showing itself to be a highly efficient, stable and versatile mRNA delivery platform for cancer immunotherapy with lower odds of toxicity. It could be stored at above freezing temperatures, and its single-macromolecule design simplifies manufacturing and supports clinical scalability.
Zhang and her team used mRNA loaded with proteins called cytokines, which can be strategically used to activate key immune responses that target tumors to limit or reverse cancer growth. This mRNA was deposited into its PFHA-PEI-mRNA-HP structure, then injected into mice with breast cancer. Not only did the mRNA reach its destination undamaged over 90% of the time, but the tumors stopped growing or even shrank.
“Its ability to achieve over 90% transfection efficiency across multiple cancer cell lines – surpassing commercial lipid-based formulations – and its demonstrated tumor suppression in a triple-negative breast cancer model when combined with anti–PD-L1 therapy, underscores its therapeutic impact,” said Zhang.
The team hopes to build upon the success of this study by investigating PFHA-PEI-mRNA-HP’s effectiveness in larger animals, as well as testing if other kinds of mRNA cargo can be delivered with this exciting new molecule.
This research was supported by the Kuni Foundation and NIH grant (R01EB026890).