By Theresa Gabrielli
September 21, 2023
Stem cell therapy represents the future of regenerative medicine. A material called chitosan may be the key to increasing the supply of clinical-grade cells.
Stem cells, characterized by their "multipotency” – their ability to transform into diverse tissue cell types – have incredible potential to address a spectrum of medical conditions such as spinal cord injuries, degenerative and autoimmune diseases, and even certain cancers. Unfortunately, stem cell therapies often involve administering billions of clinical-grade cells per patient in order to be successful, and cells in that quantity can be hard to come by.
New research coming out of the University of Washington and published this week in Matter may help alleviate these supply issues. Miqin Zhang, a professor of materials science and engineering, and her team, have designed three-dimensional scaffolds made of medical-grade chitosan that are highly effective at encouraging human neural stem cells (hNSCs) to replicate and expand under xeno-free and chemically defined conditions.
“The behavior of cells is influenced by both the material’s concentration and stiffness,” said Zhang. “Pure chitosan of high concentration promotes cell adhesion and metabolism critical for multipotency maintenance.”
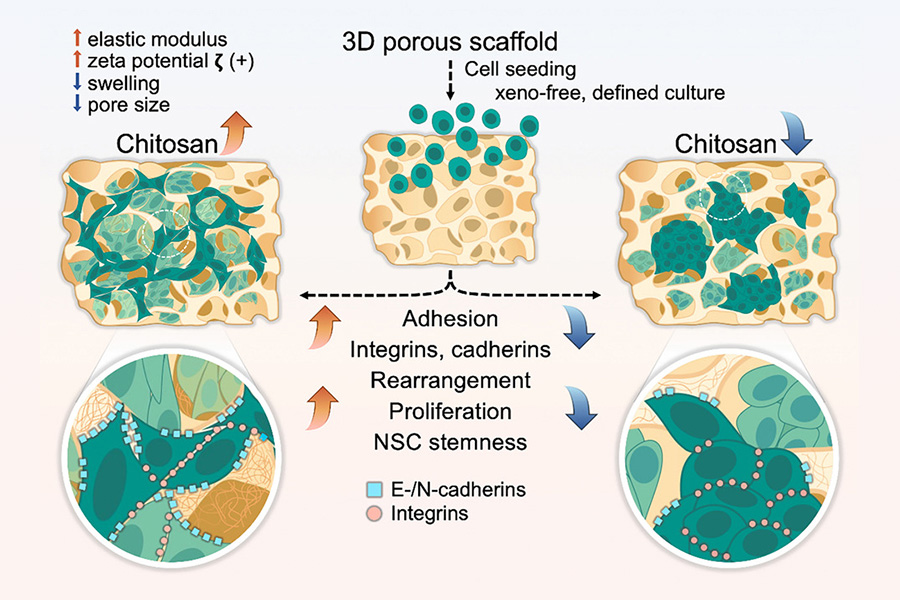
Cells are seeded into the chitosan scaffold, adhere to the inner walls of the porous structure, and expand to form cell colonies. Higher concentrations of chitosan create stiffer structures, promoting cell adhesion and proliferation. The natural properties of chitosan proved highly effective for proliferation, metabolism, and multipotency maintenance of hNSCs, without the use of animal-sourced feeder substrates.
Traditional glass or plastic Petri dishes, though widely used, are very rigid and don't resemble the body’s natural environment, hindering proper stem cell growth. These 2D culture systems also require a labor-intensive process called subculturing, demanding more resources and human involvement, and posing challenges for scaling up. Three-dimensional scaffolds, on the other hand, better mimic the environment where stem cells naturally develop within a living organism, making this a more reliable platform for cultivation. However, the choice of material for these scaffolds is critical to the process.
Chitosan, a natural polymer sourced from the hard outer skeletons of shellfish, is particularly well-suited for this purpose. Zhang and her research team constructed small, porous scaffolds with varied concentrations of chitosan, and found that those with the highest concentrations had the best results: hNSCs were able to easily adhere themselves to the porous structure inside the scaffolds and multiply. Chitosan’s natural properties boosted and protected the multipotent cell colonies while simultaneously suppressing the growth of cells with genetic variations or mutations.
Remarkably, this process was possible without relying on animal-sourced feeder substrates. Previous methods of cultivating stem cell colonies, both in 2D Petri dishes and 3D scaffolds not made with chitosan, often required the addition of these substrates to enhance cell adhesion and maintain the cells’ stemness. However, these substrates are costly and can potentially introduce pathogens or other contaminants, making those cells no longer useful in a clinical setting.
Zhang’s team believes that chitosan scaffolds have the potential to replace feeder substrates in stem cell development. Chitosan is considerably cheaper than these substrates, and its malleable nature suggests it could be adapted for use in cultivating other types of stem cells. The UW team’s process for producing scaffolds is simple and readily scalable, which means that shortages of clinical-grade stem cells could soon be a thing of the past.
“Stem cell therapy offers advantages over traditional treatments, including enhanced tissue regeneration and reduced risk of rejection. It can target diseases that currently have limited treatment options, offering hope for patients,” said Zhang.
This research was made possible through crucial support from the Kyocera Corporation.